|
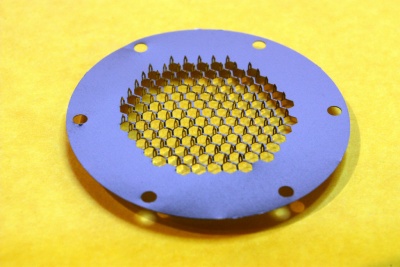
Products & Technology AdminPatch™ Microneedle Array is Refined Microneedle Technology nanoBioSciences, LLC has developed the patented Advanced micro-needle array (AdminPatch® Array) which is superior to other various microneedle technologies. The AdminPatch array painlessly and instantaneously forms hundreds of tiny aqueous channels (‘micropores’) through the stratum corneum and epidermis, the outer resistive surface layers of the skin. Numerous drugs including proteins and water-soluble molecules can enter the body through these aqueous micropores for either local effect, or by entering the circulation, for systemic effect. The created aqueous channels stay constantly open while AdminPatch array is applied on the skin and, therefore, enable the rapid, sustained, and efficient delivery of drugs through these aqueous channels formed in the skin surface. When the microneedle array is removed from the skin, the micropores simply collapse and the skin barrier is quickly restored.
AdminPatch® 777 Microneedle Array The aqueous channels formed by the microneedles in the stratum corneum using the AdminPatch® 300 and 600 systems have a depth of about 150-500 microns, sufficient to extend through the viable epidermis but shallow enough to avoid blood capillaries located deeper the dermis and any pain receptors. Longer microneedle arrays are also available for purchase. When the transdermal patch with microneedles is removed from the skin, the micropores simply collapse and the skin barrier is quickly restored. The painless AdminPatch® system combines effective delivery of drugs through the skin with excellent skin sensation and cosmetics, is easy and intuitive to use by patients and medical personnel alike, and can be economically produced to scale using mature high-volume low-cost processes.
Microneedle Array-Based AdminPen™ Liquid Injection Device AdminPen™ pen-injector device is based on our own patented proprietary microneedle array technology called AdminPatch® microneedle array. A simple low-cost molded plastic part is simply attached on the back surface of the AdminPatch microneedle array to provide a fluidic connection of AdminPen device to an externally connected liquid drug reservoir. AdminPen pen-injector device can be mounted on any commercially available injector pens with a pre-filled drug cartridge. The patented AdminPen™ device allows more effective and painless delivery of many vaccines (such as Ebola, Flu, and cancer vaccines), including particle-based vaccines, liquid medical drugs, cosmetics, and hair growth supplements uniformly into 1cm2 area of the skin. The microneedle array is formed from a metal film allowing AdminPen to be inexpensively manufactured using scalable mature high-volume, low-cost processes. AdminPen is expected to be classified by the FDA as a Class II medical device with a 510(k) regulatory approval route. AdminPen can be used with any standard commercially available pen with a pre-filled drug cartridge or with any standard commercially available syringe.
AdminPen
600 Microneedle Liquid Injection Device Competitive Advantage of AdminPen:
We are the only company capable of producing convex microneedle arrays required for reliable insertion of all microneedles into flexible skin and to deliver the already approved vaccines and drugs. The patented painless AdminPen device is an unique and superior device allowing effective delivery of all vaccines, including particle-based and DNA vaccines, liquid drugs, or injectable cosmetics uniformly into 1 cm2 area of the skin and can be inexpensively manufactured using mature high-volume, low-cost processes, and follows the 510(k) regulatory approval route. The painless AdminPen device combines effective delivery of drugs through the skin with excellent skin sensation and cosmetics, is easy and intuitive to use by patients and medical personnel alike, and can be economically produced to scale using mature high-volume low-cost processes. The Company’s strategy of applying the AdminPen tip to the existing already-approved drugs allows the Company to avoid both the costs and time spent on drug discovery and the risks of bringing a new compound to the market as well as provides it with a significant pipeline of potential products based on existing already-approved drugs.
Our AdminMed division is working on commercialization of the developed microneedle array technology. For more information and to purchase product samples please click on the following picture to visit AdminMed.com web-site: |
||
|
|
||